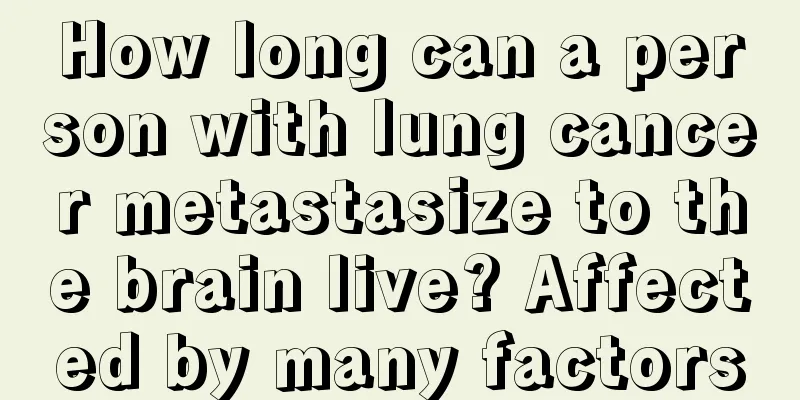pH value comparison table acidity and alkalinity

|
The pH value refers to the degree of acidity and alkalinity. Everything in life has a certain pH value, including air, food, water, etc. The acidity and alkalinity affect our lives and can also have different consequences for our lives. It is not good if the acidity and alkalinity of something are too high. The acidity and alkalinity can be measured in many ways. The most common one is the pH test paper. So what is the acidity and alkalinity of the pH value comparison table? The hydrogen ion concentration index refers to the ratio of the total number of hydrogen ions to the total amount of substance in the solution. Generally referred to as "pH" rather than "pH value". The hydrogen ion activity index can be determined by qualitative methods using pH indicators and pH test papers, while quantitative pH measurements require the use of a pH meter. Acidity and alkalinity describe the strength of the acidity and alkalinity of an aqueous solution and are expressed as pH values. Under standard thermodynamic conditions, an aqueous solution with a pH of 7 is neutral, one with a pH of <7 is acidic, and one with a pH of >7 is alkaline. The pH range is between 0 and 14 and is only applicable to dilute solutions. The acidity and alkalinity of solutions with hydrogen ion concentration or hydroxide ion concentration greater than 1 mol/L are directly expressed in terms of concentration. At 298K, when pH < 7, the solution is acidic, when pH > 7, the solution is alkaline, and when pH = 7, the solution is neutral. The acidity and alkalinity of an aqueous solution can also be measured by pOH, which is the negative logarithm of the hydroxide ion. Due to the existence of autoionization equilibrium in water, at 298K, pH + pOH = 14. A pH value less than 7 indicates that the concentration of H is greater than the concentration of OH, so the solution is highly acidic, while a pH value greater than 7 indicates that the concentration of H is less than the concentration of OH, so the solution is highly alkaline. Therefore, the smaller the pH value, the more acidic the solution is; the larger the pH value, the more alkaline the solution is. In non-aqueous solutions or non-standard temperature and pressure conditions, pH = 7 may not mean that the solution is neutral. This requires calculating the ionization constant of the solvent under such conditions to determine the neutral pH value. For example, at a temperature of 373K (100°C), the pH of a neutral solution is ≈ 6. It should also be noted that the significant figures of pH are recorded starting from the decimal point. The part before the decimal point is the exponent and cannot be recorded as a significant figure. |
<<: How long does it take to recover after pterygium surgery
>>: Will pterygium disappear on its own?
Recommend
What should patients with lymphoma pay attention to
The incidence of lymphoma is relatively high. The...
What is the reason why the thumb nail turns white
Some people will find white spots or whitening on...
Is it better to have a side-suction or top-suction range hood?
The range hood is a must-have in the kitchen, as ...
What tea should I drink if I have a bad stomach and bad breath?
In life, tea is a very good health product. Drink...
Benefits of eating ugly oranges during pregnancy
Orange is a common fruit in daily life. It is del...
There is an abscess under my armpit
Many people have experienced the growth of a pust...
What risk factors increase the incidence of colon cancer?
What risk factors increase the incidence of colon...
Drink five kinds of soup to get rid of facial spots
Among natural foods, there are many kinds of food...
What are the symptoms of recurrence of colorectal cancer
What are the symptoms of colorectal cancer recurr...
Acute massive bleeding from bladder cancer
If there is heavy bleeding in the late stage of b...
Signs of death from lung cancer in the elderly
Symptoms of lung cancer before death generally in...
How long after a meal is the best time to brush your teeth?
As people in modern society have higher and highe...
What should you pay attention to when recovering from nasopharyngeal carcinoma
Radiotherapy is the first choice for NPC patients...
What to do if you have a stuffy nose due to a cold
In daily life, if you catch a cold, it may someti...
What are the symptoms of patients with advanced lung cancer before death? Pay attention to these symptoms of advanced lung cancer near death
Lung cancer can be divided into many types, but n...